MedFriendly®


Cation
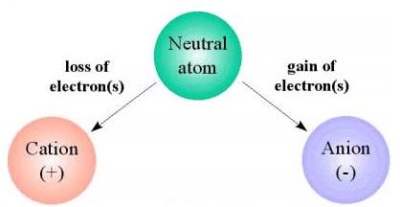
A cation is an ion with a positive electric charge.
An ion is an atom or a group of atoms that have an
electric charge by gaining or losing one or more
electrons. An atom is the smallest part of a
substance that can exist alone or in combination
with something else. An electron is a negatively
charged particle that is smaller than an atom.
FEATURED BOOK: Anion Coordination Chemistry
Since an electron has a negative charge, if an atom has too few electrons it is
considered positive (because there will be more positive charges than negative
charges). Hydrogen creates the only cation without electrons. Cations that do retain
one or more electrons are smaller than the neutral atoms or molecules from which they
come from.
Cations travel towards a negative pole known as a cathode. A pole is one of two points
that are at the extremes (for example, the positive and negative poles). Examples of
cations are the following elements: sodium, potassium, magnesium, hydrogen, and
calcium. See the entry for ions, to learn how cations are important to the body and how
abnormal levels of cations can cause problems. Compare cations to anions, which are
ions with negative electrical charges. Cationic means pertaining to cations. Cation
comes from the Greek word “kata” meaning “down” and the Greek word “ion” meaning
“going.” Put the words together and you get “going down” which is a reference to the loss
of electrons.
"Where Medical Information is Easy to Understand"™