MedFriendly®


Ion
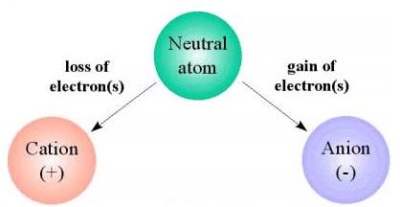
An ion is an atom or a group of atoms that have an
electric charge by gaining or losing one or more
electrons. An atom is the smallest part of a
substance that can exist alone or in combination
with something else. An electron is a negatively
charged particle that is smaller than an atom.
FEATURED BOOK: Anion Coordination Chemistry
Since an electron has a negative charge, if an atom gains too many electrons it is
considered negative (because there will be more negative charges than positive
charges). If an atom has too few electrons it is considered positive (because there will be
more positive charges than negative charges).
Ions that are charged with positive electricity are known as cations and travel towards a
negative pole known as a cathode. A pole is one of two points that are at the extremes
(for example, the positive and negative poles). Ions that are charged with negative
electricity are known as anions and travel towards a positive pole known as an anode.
This is a good example of the phrase "opposites attract."
WHAT ARE SOME EXAMPLES OF CATIONS AND ANIONS?
Examples of cations (positively charged ions) are the following elements: sodium,
potassium, magnesium, hydrogen, and calcium. Examples of anions (negatively charged
ions) are phosphate and bicarbonate. Bicarbonate is a substance in the blood that
prevents it from becoming too acidic or too alkaline (non-acidic).
"Where Medical Information is Easy to Understand"™
HOW ARE IONS IMPORTANT TO THE BODY?
Many important processes that occur in the body depend on the
movement of ions across the outer surfaces of cells. Cells are the
smallest, most basic units of life, that are capable of existing by
themselves. As an example, when the positively charged ions,
sodium and potassium, move across the outer surfaces of cells
near muscle and nerve cells, impulses are transmitted which allow
muscle contractions to occur. Calcium also plays an important part
in muscle contraction, and it also helps bone grow and helps blot
clot (come together). Sodium is the main positively charged ion that
is present in the fluid that surrounds cells.
Sodium affects the flow of water into and out of cells.
The kidney helps to control the amount of sodium, calcium, and potassium that is lost from the body
through urinating. The kidneys are two organs located on each side of the spine, behind the stomach. The
kidneys filter (remove) wastes from the blood. Hormones also affect the level of calcium in the body.
Hormones are natural chemicals produced by the body and released into the blood that have a specific
effect on tissues in the body.
The acid level of the blood and other fluids in the body are determined by the amount of hydrogen cations
that are produced through metabolism (the chemical actions in cells that release energy from nutrients or
use energy to create other substances). A hydrogen cation is a hydrogen atom that is missing its electron.
Hydrogen is a type of element. If not interfered with, hydrogen cations would cause body fluids such as
blood and the fluid that surround cells to be too acidic. However, in healthy people, negative ions known
as bicarbonate anions and phosphate anions prevent hydrogen cations from doing so.
WHAT KIND OF PROBLEMS CAN EXIST WITH IONS?
For normal body functioning to occur, ion levels need to be maintained at normal limits. Any significant
change in the level of ions in the body can cause symptoms such as muscle weakness from too low of an
amount of potassium cations in the blood. When there is too low of an amount of water in the body (which
can happen from sweating too much, for example) there can be a high level of ions in the body compared
to the amount of water that is left. This can cause symptoms such as muscle cramps, dizziness, thirst, and
a faint feeling.
CAN IONS EXIST IN LIQUIDS, SOLIDS, AND GASES?
Yes. Ions can exist in the state of a solid, liquid, or gas. Ions that exist in liquid (such as electrolytes) are
more common.
WHAT IS THE ORIGIN OF THE TERM "ION"?
Ion comes from the Greek word "ion" meaning "going."