MedFriendly®


Osmolality
BASIC TERMS NEEDED TO UNDERSTAND
OSMOLALITY?
To understand what osmolality is, it is first necessary to
understand a few other terms. If you already know the
following terms, feel free to skip to the next section.
SOLVENT: A usually liquid substance (such as water)
that is capable of dissolving or dispersing one or more
substances.
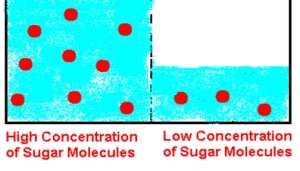
High osmolality on the right but
low osmolality on the left.
FEATURED BOOK: Mosby's Diagnostic and Laboratory Test Reference
SOLUTE: A substance (such as sugar) that dissolves in a solvent. So if you poured
sugar into a cup of water, the sugar would be the solute and the water would be the
solvent.
SOLUTION: The combination of a solute with a solvent, when the solute has been evenly
dissolved in the solvent. An example would be when sugar (the solute) is evenly dissolved
in a glass of water (the solvent).
CONCENTRATION: A measurement of the amount of a solute compared to the amount of
solvent in a solution. As an example, if a high amount of sugar (the solute) is dissolved in
a small amount of water (the solvent), the concentration would be high. The concentration
would be low if a small amount of sugar was dissolved in a large amount of water.
"Where Medical Information is Easy to Understand"™
SEMIPERMEABLE MEMBRANE: A thin layer of tissue that allows
some substances in, but not all. For example, the semipermeable
membrane may allow a smaller substance in but not a larger one. Or
it may only allow the solvent (such as water) to pass through, but
not allow any solutes to pass through. Semipermeable membranes
are widespread in the body and surround all cells.
WHAT IS OSMOLALITY?
Osmolality is the concentration of a solution. It is a measurement of
the amount of a solute compared to the amount of solvent in a
solution. See above for a description of some of these terms.
HOW IS OSMOLALITY EXPRESSED IN TERMS OF MEASUREMENT?
Osmolality is expressed in units of measurement known as osmoles or milliosmoles (one thousandth of an
osmole), per 1000 grams (a very small unit of weight) of solvent. An osmole is the weight of the molecules
(the smallest naturally occurring particles of a substance) that make up a solute, divided by the number of
particles or ions (electrically charged particles) that they separate in a solution. The molecules that make
up the solute are usually measured in grams.
WHAT IS THE NORMAL RANGE OF OSMOLALITY IN BLOODWORK TESTS?
Osmolality can be measured in the blood. The normal range for adults is 285 to 295 milliosmoles
(abbreviated as mOsm) divided per kilograms (one thousandth of a gram) of water. However, some
hospitals use a range of 280-300.
WHAT DOES IT MEAN IF THE OSMOLALITY IS TOO HIGH?
Osmolality can be too high for several reasons. One reason is a loss of too much water or other types of
fluids from the body. Osmolality would be too high because there would be less liquid in comparison to the
amount of solute (something dissolved in the liquid). Another cause would be the presence of too much
salt or sugar in the blood. Uremia (a condition in which substances build up in the blood that are normally
passed out of the body when one pees) can also cause a high osmolality level. The presence of
poisonous levels of certain substances in the blood, such as alcohol, can cause a high osmolality level.
The presence of the drug, Mannitol, which is a type of sugar that the body has difficulty breaking down,
can lead to a high osmolality level because it can build up in the blood. Diabetes insipidus, which is a
disease that causes too much fluid to leave the body, can also lead to a high osmolality level.
WHAT DOES IT MEAN IF THE OSMOLALITY IS TOO LOW?
Too much water or fluid in the body can cause a low osmolality level. This is because there is too much
liquid in comparison to the amount of substances (solutes) that are dissolved in it. To low of an amount of
salt in the blood can cause a low osmolality level. Also, a condition known as SIADH (Syndrome of
Inappropriate Diuretic Hormone) can cause a low osmolality level. SIADH is an abnormal condition in
which the body releases too much of a substance called antidiuretic hormone. Antidiuretic hormone works
to keep water in the body. Thus, if there is too much antidiuretic hormone released, there will be too much
fluid in the body.